According to media reports on Wednesday, Chinese regulators have approved clinical trials on the efficacy and safety of local mRNA boosters for inactivated vaccines.
The initiative has been approved by the Guangxi Zhuang Autonomous Region’s disease control and prevention center in South China. According to an announcement on the Ministry of Science and Technology’s website, it will focus on patients aged 18 and up who have received two inactivated vaccination doses.
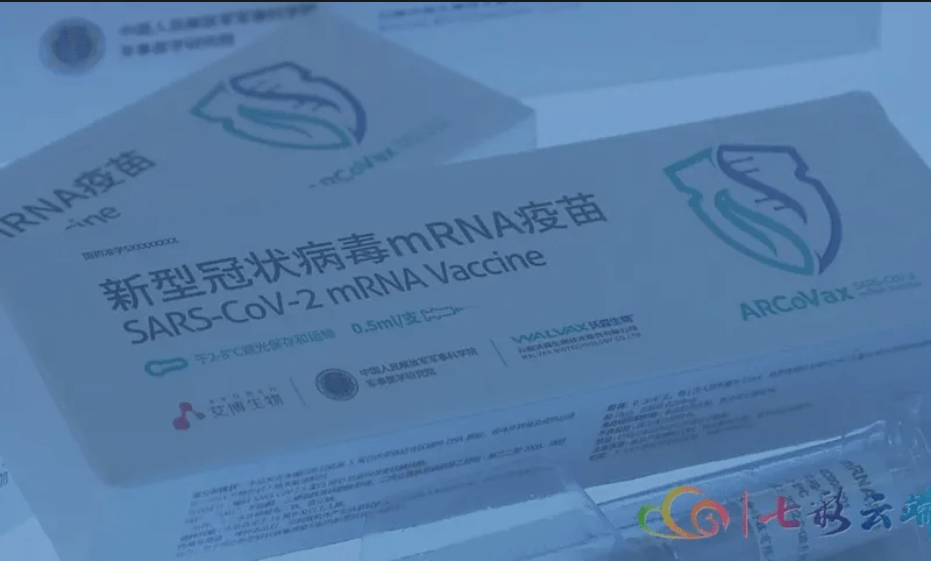
According to media reports on Wednesday, the mRNA candidate shot to be used in the trials is named ARCoVax, and it was developed in collaboration with the Academy of Military Medical Sciences, Suzhou Abogen, and Yunnan Walvax Biotechnology Co.
According to Zhang Li, a spokesman for Walvax, the project is part of ARCoVax’s Phase III clinical trials, which are taking place in Guangxi and other countries such as Mexico and Indonesia.
The project’s announcement raised Walvax’s stock by around 13% on Wednesday afternoon.
Observers are also anticipating the approval of the first domestic mRNA vaccine in the near future.
Suzhou Abogen, Walvax’s partner, said on Saturday that their mRNA vaccine production line in a Suzhou industrial park had received official approval. The plant has a capacity of 40 million shots per year.